Fully Exposed Pd Clusters for Low-Temperature Direct Dehydrogenation Reaction
Direct dehydrogenation of alkanes is of great interest but presents a significant challenge in activating C-H bond at a low temperature. Styrene (ST), as an important monomer, is widely used in the production of engineering plastics, rubber and resin. At present, vast amounts of overheated steam and up to 620 °C reaction temperature were strictly required to obtain a satisfactory ST yield in the DDH process due to the endothermic nature of the reaction, resulting in high energy and water consumption. Apart from promoting the conventional iron oxide-based catalysts by incorporating different promoters, novel nanocarbons as metal-free catalysts were investigated to boost DDH activity and anticoking ability. However, the reaction temperature in these studies was still as high as 550 °C, resulting in limited relief of energy consumption. Thus, it is still urgent to develop novel catalysts with promising low-temperature activity for EB DDH reaction.
Recently, Research Scientist LIU Honyang, Research Associate DIAO Jiangyong, and PhD Student WANG Linlin at Shenyang National Laboratory for Materials Science cooperated with Professor MA Ding at Peking University, Professor WANG Ning at the Hong Kong University of Science and Technology, Research Scientist JIANG Zheng at Shanghai Institute of Applied Physics, CAS, Research Scientist WEN Xiaodong at Institute Coal Chemistry, CAS, and their teams, to obtain fully exposed Pd clusters consisting of an average of three Pd atoms on a hybrid nanodiamond-graphene support (denoted as Pd3/ND@G) for low-temperature dehydrogenation of ethylbenzene to styrene, which exhibit good catalytic performance and much enhanced stability under oxygen-free conditions at 350 °C.
Research Scientist LIU Hongyang and his team have dedicated years into the study of precise design of metal catalysts at atomic scale and efficient utilization of alkanes (Nat. Commun. 12(2021)2664, Nat. Commun. 10(2019)4431, J. Am. Chem. Soc. 141(2019)18921, J. Am. Chem. Soc. 140(2018)13142, ACS Cent. Sci. 7(2021)262, ACS Catal. 9(2019)5998). Building on the previous studies, the research team successfully constructed fully exposed Pd clusters with atomic dispersion anchoring onto the ND@G support. As evidenced by aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (Fig.1) and X-ray absorption fine structure (Fig.2), the fully exposed Pd clusters with an average atomicity of 3 are stabilized on the defect-rich graphene surface via Pd-C bonds. The as-prepared fully exposed Pd clusters showed much higher catalytic activity (328 mmol?gPd-1?h-1 EB conversion rate) and selectivity toward ST (>99%) than Pd SACs and Pd nanoparticles (NPs) for the DDH of EB at 350℃. Density functional theory (DFT) calculation results (Fig.4) indicated that the synergetic interaction among adjacent Pd atoms in each cluster with limited size guarantees effective activation of C-H bond on ethyl in EB and easy desorption of ST, leading to the remarkable low-temperature EB DDH performance over the Pd3/ND@G catalyst. The present method for precise control of fully exposed Pd clusters paves a new way for designing direct dehydrogenation catalyst at the atomic scale.
This work was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China, DNL Cooperation Fund, CAS, the Liaoning Revitalization Talents Program, the Sinopec China, the Young Talent Program of Shenyang National Laboratory for Materials Science, the Research Grants Council of Hong Kong. The XAS experiments were conducted in Shanghai Synchrotron Radiation Facility (SSRF).
The paper was recently published on line by ACS Catalysis, https://doi.org/10.1021/acscatal.1c01503
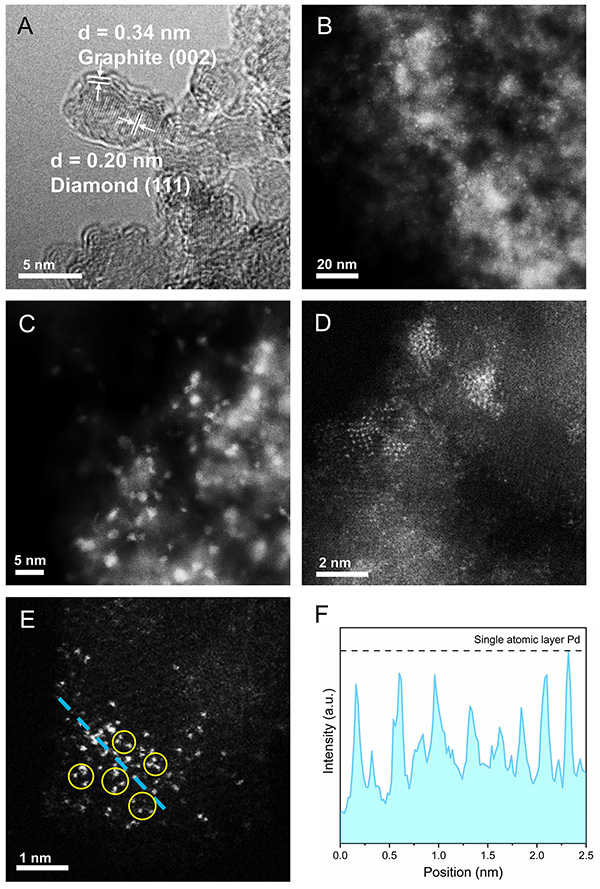
Fig.1. (A) HRTEM image of ND@G nanocarbon support. AC-HAADF-STEM image of Pd3/ND@G at low (B, C) and high (D, E) magnifications (typical fully exposed Pd clusters are highlighted by the yellow circles in E). (F) Extracted line profile along the blue dashed line in(E), demonstrating the single-atomic-layer thickness of Pd clusters.
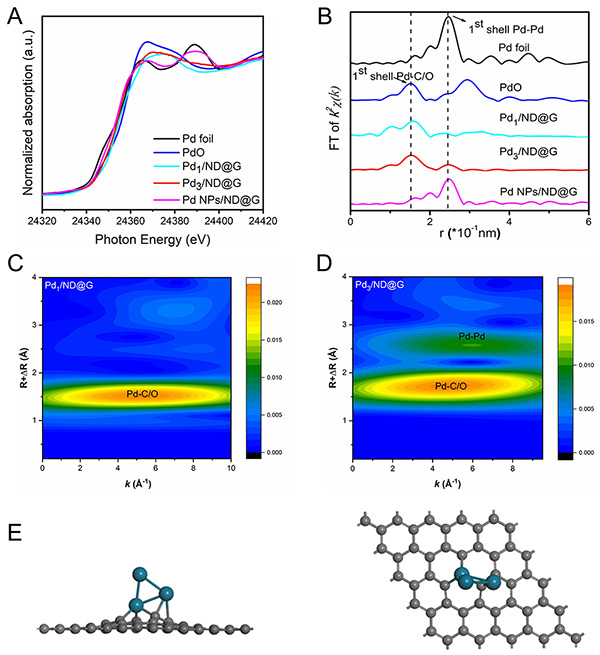
Fig. 2. Pd K-edge XANES profiles (A) and extended X-ray absorption fine structure (EXAFS) spectra (B) in R space for Pd1/ND@G, Pd3/ND@G, Pd NPs/ND@G, Pd foil, and PdO. Wavelet transform (WT) analysis of Pd1/ND@G (C) and Pd3/ND@G (D). (E) Optimized structure of Pd atom embedded into graphene from side (left) and top (right) views, generated from EXAFS fitting results.
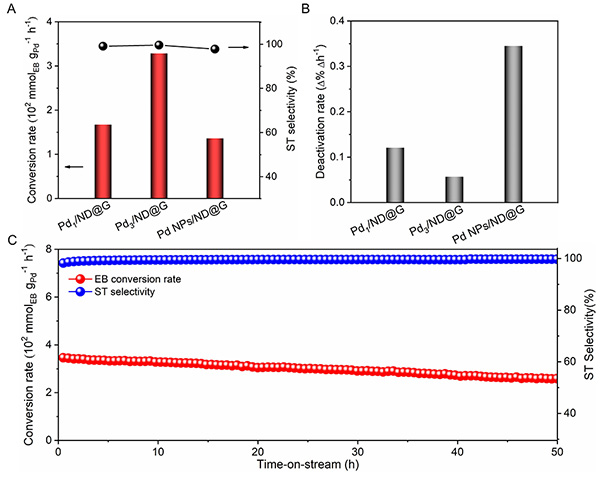
Fig 3. Ethylbenzene dehydrogenation reactivity (A) and deactivation rate (B) of different catalysts (data from 10 h steady-state reactivity). (C)Long-term stability of Pd3/ND@G on dehydrogenation of ethylbenzene.
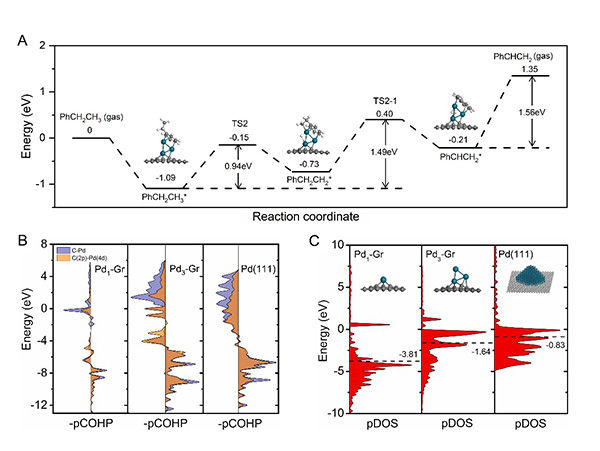
Fig 4. (A) Calculated energy profile of ethylbenzene dehydrogenation to styrene. The projected crystal orbital Hamilton population (pCOHP) curves (B) for Pd 4d states and projected density of states (pDOS) plots (C) of C-Pd bond for styrene molecules adsorbed of Pd1-Gr, Pd3-Gr, and Pd (111) surfaces.
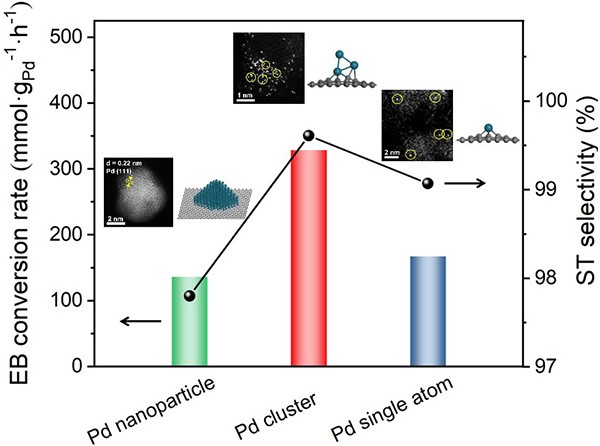
Fig.5 EB conversion rates and ST selectivity of different Pd active centers at atomic scale.